Our Research
Our Research Interests
 Our lab is interested in exploring the roles of the Hippo signaling network in the initiation, progression, metastasis, and chemotherapeutic drug resistance of breast and lung cancers. The Hippo pathway was originally discovered in Drosophila and later in mammals. It plays critical roles in various biological processes including organ size control, tissue regeneration, tumorigenesis, and stem cell renewal and differentiation, etc.
Our lab is interested in exploring the roles of the Hippo signaling network in the initiation, progression, metastasis, and chemotherapeutic drug resistance of breast and lung cancers. The Hippo pathway was originally discovered in Drosophila and later in mammals. It plays critical roles in various biological processes including organ size control, tissue regeneration, tumorigenesis, and stem cell renewal and differentiation, etc.
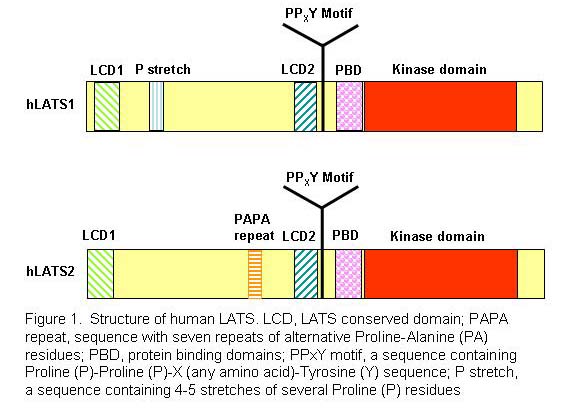
Specifically, we are studying the roles of the core Hippo components including LATS1/2 and YAP and its paralog TAZ in cancer. LATS (Large Tumor Suppressor)/warts is the founding member of the Hippo pathway and a serine/threonine kinase (Figure 1) originally isolated in Drosophila (fly) as a cell proliferation inhibitor (Xu et al., 1995). There are two mouse and two human homologs of fly LATS, LATS1 and LATS2. While LATS2 knockout mice are lethal, mice deficient for LATS1 develop soft-tissue sarcoma, ovarian tumors, and pituitary dysfunction. We provided the first biological evidence that LATS1 is a tumor suppressor in humans (Yang et al., 2001) and identified YAP transcriptional co-activator as the first substrate and downstream target of LATS1 (Hao et al., 2008). We also identified HxR/K/HxxS/T as the consensus phosphorylation site of LATS1/2 kinase (Hao et al., 2008).
LATS and Hippo Pathway in Cell Cycle Regulation and Tumorigenesis
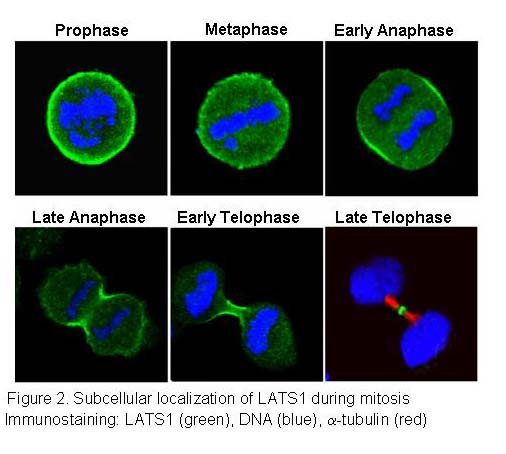 We previously showed that human LATS1 can suppress human tumor cell growth by causing G2/M cell cycle arrest through inhibition of Cdc2/Cyclin B activity (Yang et al., 2001). LATS1 is localized at the contractile ring or cleavage furrow during mitosis and cytokinesis (Figure 2). Most importantly, inactivation of LATS1 in cells or gene knockout in mice causes enhanced cytoskeletal actin polymerization, abrogates cytokinesis,resulting in chromosome instability, which is an important cancer marker (Yang et al., 2004). Similar to LATS1, overexpression of LATS2 induces either G1/S or G2/M arrest and loss of LATS2 also cause cytokinesis defects and chromosome instability. We are also exploring the roles of LATS and other Hippo pathway components in the regulation of cell cycle progression and tumorigenesis
We previously showed that human LATS1 can suppress human tumor cell growth by causing G2/M cell cycle arrest through inhibition of Cdc2/Cyclin B activity (Yang et al., 2001). LATS1 is localized at the contractile ring or cleavage furrow during mitosis and cytokinesis (Figure 2). Most importantly, inactivation of LATS1 in cells or gene knockout in mice causes enhanced cytoskeletal actin polymerization, abrogates cytokinesis,resulting in chromosome instability, which is an important cancer marker (Yang et al., 2004). Similar to LATS1, overexpression of LATS2 induces either G1/S or G2/M arrest and loss of LATS2 also cause cytokinesis defects and chromosome instability. We are also exploring the roles of LATS and other Hippo pathway components in the regulation of cell cycle progression and tumorigenesis
The Roles of the Hippo Pathway in Apoptosis and Response to Chemotherapeutic Drug Treatments
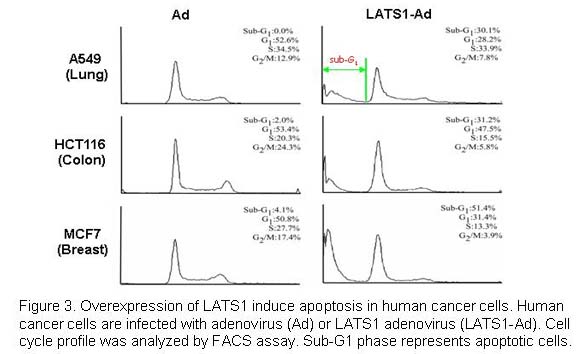 We have previously shown that overexpression of LATS1 induces apoptosis in various human cancer cells by activation of p53 and caspase-3 activity (Figure 3; Yang et al., 2001). Recently, we also identified a novel signaling pathway, Cdk1-YAP/TAZ-TEAD-CTGF/Cyr61, which is involved in Taxol drug resistance in breast cancer (Lai et al., 2011; Zhao et al., 2005a,b). To find effective therapies for drug-resistant breast and lung cancers, we are currently exploring how the Hippo pathway is involved in resistance of cancers to many other chemotherapeutics. Our research findings will provide valuable therapeutic strategies for future successful treatment of human cancers.
We have previously shown that overexpression of LATS1 induces apoptosis in various human cancer cells by activation of p53 and caspase-3 activity (Figure 3; Yang et al., 2001). Recently, we also identified a novel signaling pathway, Cdk1-YAP/TAZ-TEAD-CTGF/Cyr61, which is involved in Taxol drug resistance in breast cancer (Lai et al., 2011; Zhao et al., 2005a,b). To find effective therapies for drug-resistant breast and lung cancers, we are currently exploring how the Hippo pathway is involved in resistance of cancers to many other chemotherapeutics. Our research findings will provide valuable therapeutic strategies for future successful treatment of human cancers.
Roles of Hippo Pathway in Cytoskeletal Organization and Cancer Cell Migration
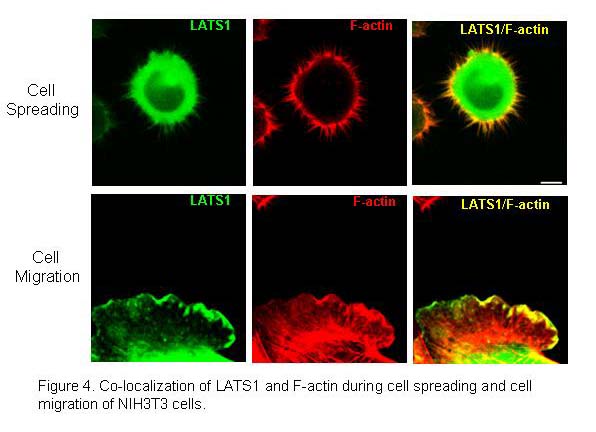 Our previous studies show that LATS1 is colocalized with F-actin at the actomyosin contractile ring during cytokinesis and the localization at the ring is actin-dependent (Yang et al., 2004). Loss of LATS1 causes enhanced actin polymerization at the contractile ring. In addition, our previous studies have also demonstrated that LATS1 can bind to and inhibit actin-binding protein LIMK1, a central player in Rho/Rac/Cdc2 signal pathway that regulates actin polymerization, cytoskeletal organization, cell migration and invasion. Further we have recently found that LATS1 co-localizes with filament (F)-actin during cell spreading and cell migration (Figure 4)(Visser et al., 2011). Most importantly, we found loss of LATS causes increased cell migration and adhesion (Visser and Yang, 2010; Visser et al., 2011). Moreover, we have also demonstrate that LATS substrates YAP and TAZ also play important roles in regulating epithelial-mesenchymal transition and cell migration through regulation of deltaNp63 and BMP4 (Lai et al., 2011; Valencia et al., 2005).
Our previous studies show that LATS1 is colocalized with F-actin at the actomyosin contractile ring during cytokinesis and the localization at the ring is actin-dependent (Yang et al., 2004). Loss of LATS1 causes enhanced actin polymerization at the contractile ring. In addition, our previous studies have also demonstrated that LATS1 can bind to and inhibit actin-binding protein LIMK1, a central player in Rho/Rac/Cdc2 signal pathway that regulates actin polymerization, cytoskeletal organization, cell migration and invasion. Further we have recently found that LATS1 co-localizes with filament (F)-actin during cell spreading and cell migration (Figure 4)(Visser et al., 2011). Most importantly, we found loss of LATS causes increased cell migration and adhesion (Visser and Yang, 2010; Visser et al., 2011). Moreover, we have also demonstrate that LATS substrates YAP and TAZ also play important roles in regulating epithelial-mesenchymal transition and cell migration through regulation of deltaNp63 and BMP4 (Lai et al., 2011; Valencia et al., 2005).
Since cell migration play critical roles in cancer metastasis and over 90% cancer patients die of tumor metastasis, our research findings on the roles of the Hippo pathway components in cytoskeletal organization and cell migration will shed light on our understanding not only the basic biology of cell migration but also the molecular mechanism of metastasis during cancer development.







